Article by Karlee De Monnin
CASE
During my first ambulance ride-along as a fourth-year medical student on an EMS elective, my unit responded to a call for abdominal pain. On arrival to the apartment building, we stepped inside to find a tearful, but otherwise well-appearing young woman lying on the floor of her living room.
She was an otherwise healthy 23-year-old who called us for abdominal pain and vomiting. She noted a positive home pregnancy test several days prior, for which she hadn’t yet sought prenatal care. Her abdominal pain, localized to the left lower quadrant, had been present for several days, but she had become severely nauseated and vomited several times over the past hour since awakening that morning. The paramedics assisted the patient into the ambulance. Her vitals were stable, and the team placed an IV. We began transport to the hospital.
With the truck in motion, the paramedic focused his attention on his computer screen, asking the patient several questions and inputting her information. All the while, sweat began to bead up along the patient’s hairline. Glancing up, the paramedic commented on her increasing clamminess and turned on the air conditioning before turning back to his computer.
I was focused on exploring the truck as it was my first time transporting a patient in an ambulance. As seconds passed, the patient began squirming in her seat and moaning. This quickly progressed to dry heaving. At this point, the paramedic jumped up from his seat, lunged over the patient to slide open a cabinet above my head and obtained an emesis bag. The bag made it just in time. “We’re out of Zofran, unfortunately,” he murmured to me.
REFLECTION
At first glance, this might appear to have been a relatively smooth encounter. We picked up a patient, evaluated her, and transported her safely to the hospital. And yet, there’s a tiny detail in this case that highlights a potential area for improvement: this patient had established a pattern of vomiting prior to our arrival on scene, of which we were fully aware. In the rig, she started showing clear signs that she was about to have another episode of emesis, and yet, we failed to get her an emesis receptacle until the very last second, just before it was too late. We lacked appropriate situational awareness, and narrowly avoided the consequences.
Situational awareness, of which anticipation is a major component, relies to a large degree on experience. This is a skill that is built upon time and mistakes – the team in the back of the truck that day was comprised of a relatively newer paramedic, and me, a medical student riding along in an ambulance for the first time. I was in a completely new environment and experienced the consequences of an elevated cognitive burden. Neither of us anticipated emesis until it was almost too late.
To a team of more experienced prehospital clinicians, it may have been second nature to reach for an emesis bag upon entry to the vehicle. There is no substitute for experience. Yet, regardless of experience level, it is important to consider how EMS education can teach situational awareness. This may be critically important in complicated clinical scenarios, such as being able to predict the trajectory of an unstable patient and intervene appropriately, or being able to recognize a lack of scene safety and take appropriate precautions before an accident happens. The skill of anticipation is broadly applicable across prehospital care, and is likely to be amplified in stressful situations, during which a person’s cognitive load is increased.
Since it is impossible to staff all response vehicles with only the most experienced of providers, how can we best prepare newer prehospital clinicians?
LITERATURE REVIEW
Does research show situational awareness can be taught or can it only be gained through experience? In the hospital, situational awareness is an important factor in human error analysis, a critical aspect of healthcare, given that medical errors are a remarkably common cause of death. There is some literature on situational awareness across clinical settings, and a small number of studies in the prehospital setting, which can be loosely extrapolated to offer insight into effective EMS training.
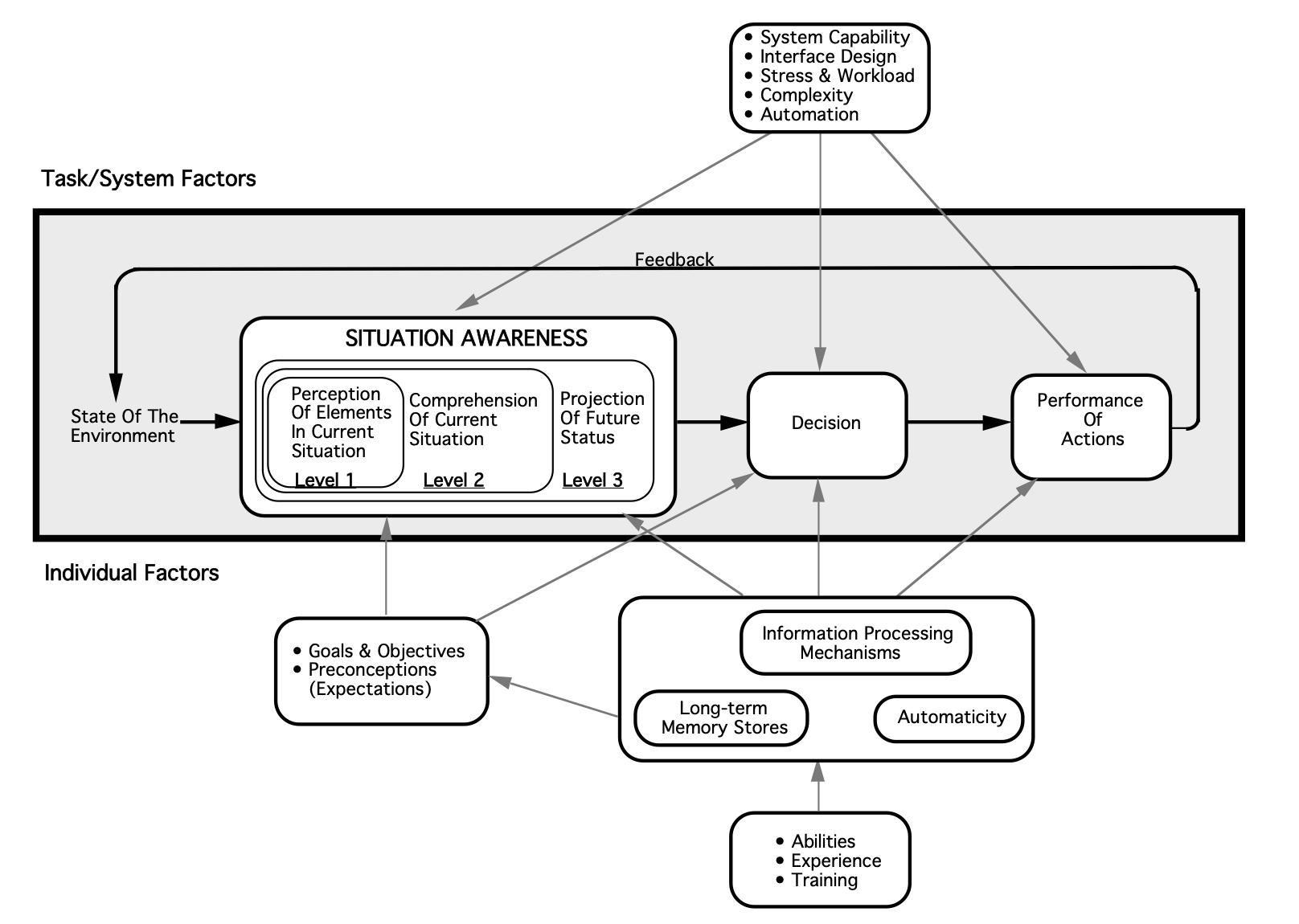
From a research perspective, the concept of situational awareness was initially developed in the aviation sector, but has since been applied broadly to other systems, including healthcare (Williams 2013). Justin Hunter and colleagues (2019) describe situational awareness as “being aware of what is happening around you, understanding what that information means now, and predicting what it will mean in the future.” In the example above, situational awareness might then be explained as recognizing the initial chief complaint of vomiting and abdominal pain, along with the patient’s increasing diaphoresis and discomfort en route.
Expanding on their definition above, Hunter and colleagues (2019) offer several theoretical frameworks by which situational awareness can be understood, of which they argue that Mica Endsley’s three-level framework of situational awareness (comprised of identification, interpretation, and prediction) is most applicable to paramedicine. Because situational awareness is a form of cognitive processing, it is unsurprisingly difficult to quantify and study. Hunter and colleagues have attempted to put forth additional studies on the topic of situational awareness, using Endsley’s framework as a guide. For example, they conducted a pilot study (2021) which considered the situational awareness of paramedic students during a simulation. Situational awareness was low and a common theme identified was the lack of an organized approach to the situation. Inability to achieve “prediction” level situational awareness was limited also by stress and failure of recognition/perception.
Image from Endsley, M. R. (1995). Toward a theory of situation awareness in dynamic systems. Human factors, 37(1), 32-64.
A small number of validated techniques have been employed for measuring situational awareness in healthcare. For example, Endsley developed the Situation Awareness Global Assessment Technique (SAGAT), which has been employed to evaluate trauma skills among medical trainees (Hogan 2006) and recognition of patient deterioration among student nurses (Kinsman 2009, Cooper 2019). This test requires halting a simulated scenario to assess participant insight into situational awareness, utilizing standardized reflective questions. An alternative metric, the Situation Present Assessment Method (SPAM), has also been employed (Williams 2013). Additionally, some interest has been expressed in examining eye tracking as a means of measuring situational awareness, though the efficacy of this, as well as its utility in clinical education, remains to be seen (Williams 2013). Little of this research has touched the prehospital arena.
Within the prehospital setting, Hunter’s group has recently started to examine educational strategies to modify situational awareness, with a statistically significant increase in situational awareness with an educational intervention in a quasi-experimental before-after study in 2022. The educational tool employed by Hunter and colleagues focused on elements of Endsley’s situational awareness model and some adapted principles from aviation, Crew Resource Management, designed to improve situational awareness.
Though the study is small, there is something to be said for acknowledging the potential value of education on situational awareness in paramedic training. The limited research on this topic is illustrative of how difficult it is to quantify and study. In the absence of clear, compelling evidence, an important question arises as to how medical directors can identify the need for targeted teaching and feedback of nontechnical skills like this, as they will not necessarily be reflected on chart review, and whether there is even value in broadly incorporating education on this skill.
CONCLUSION
Situation awareness education and training for prehospital clinicians and scenarios needs additional investigation. As research continues, we may discover evidence for interventions to improve situational awareness. Although there are time and financial costs to education and training, it important to remember that situational awareness itself is critical to prehospital patient care.
REFERENCES
Cooper S, Kinsman L, Buykx P, McConnell-Henry T, Endacott R, Scholes J. Managing the deteriorating patient in a simulated environment: nursing students' knowledge, skill and situation awareness. J Clin Nurs. 2010;19(15-16):2309-2318. doi:10.1111/j.1365-2702.2009.03164.x
Hogan MP, Pace DE, Hapgood J, Boone DC. Use of human patient simulation and the situation awareness global assessment technique in practical trauma skills assessment. J Trauma-Injury Infect Crit Care. 2006;61(5):1047–1052.
Hunter, J., Porter, M., & Williams, B. (2019). What Is Known About Situational Awareness in Paramedicine?: A Scoping Review. Journal of allied health, 48(1), e27–e34.
Hunter, J., Porter, M., Phillips, A., Evans-Brave, M., & Williams, B. (2021). Do paramedic students have situational awareness during high-fidelity simulation? A mixed-methods pilot study. International emergency nursing, 56, 100983. https://doi.org/10.1016/j.ienj.2021.100983
Hunter, J., Porter, M., Cody, P., & Williams, B. (2022). Can a targeted educational approach improve situational awareness in paramedicine during 911 emergency calls?. International emergency nursing, 63, 101174. https://doi.org/10.1016/j.ienj.2022.101174
Kinsman, L., Endacott, R., Cooper, S. J. R., Scholes, J., Buykx, P., & McConnell-Henry, T. E. (2009). Situational awareness of patient deterioration in a simulated environment.
Williams, B., Quested, A., & Cooper, S. (2013). Can eye-tracking technology improve situational awareness in paramedic clinical education?. Open access emergency medicine : OAEM, 5, 23–28. https://doi.org/10.2147/OAEM.S53021
About the Author:
Karlee is an emergency medicine-bound fourth-year medical student at Washington University in St. Louis. She has a strong clinical interest in medical education. Outside the hospital, she can be found running with her dog, baking, or getting lost in a book.
Editing by James Li, MD