by Torie Howarth, NRP and Joshua Stilley, MD FAEMS
Scenario
You are dispatched for a 78 year old female with a complaint of generalized weakness. On arrival, the patient is sitting in her bedroom and appears pale but alert. You conduct a quick primary survey and find that while her radial pulse is present, you only count 5 beats over 10 seconds (30!).
You and your partner quickly apply the patches and monitor:
The 12 lead shows:
Her full set of VS is P 36, BP 96/50, RR 26, SpO2 98% and EtCO2 of 12.
You establish an IV and administer 0.5 mg of atropine. The heart rate momentarily climbs to 45 without an improvement in signs of perfusion. At that point you elect to initiate pacing, but are unable to achieve capture even with maximum settings. You decide to get some more information from the patient and her family.
The family reports that the patient has a history of hypertension. She was seen by her primary care doctor last week and who increased her diltiazem dose. For the last few days she has been feeling unwell, and eating and drinking less. This morning she was too weak to get out of bed and minimally conversant, prompting them to call 911.
What is going on?
Cognitive Detour:
Daniel Kahneman, PhD has published literature describing what he has termed System 1 and System 2 thinking. [1] System 1 is the brain’s automatic, intuitive and unconscious mode. System 2 is slow, deliberate, and conscious. It is where the critical thinking and reasoning dominates.
The shortcuts that System 1 thinking makes are also known as the use of heuristics. The definition of a heuristic is:
“simple, efficient rules which people often use to form judgments and make decisions. They are mental shortcuts that usually involve focusing on one aspect of a complex problem and ignoring others.” (Wikipedia)
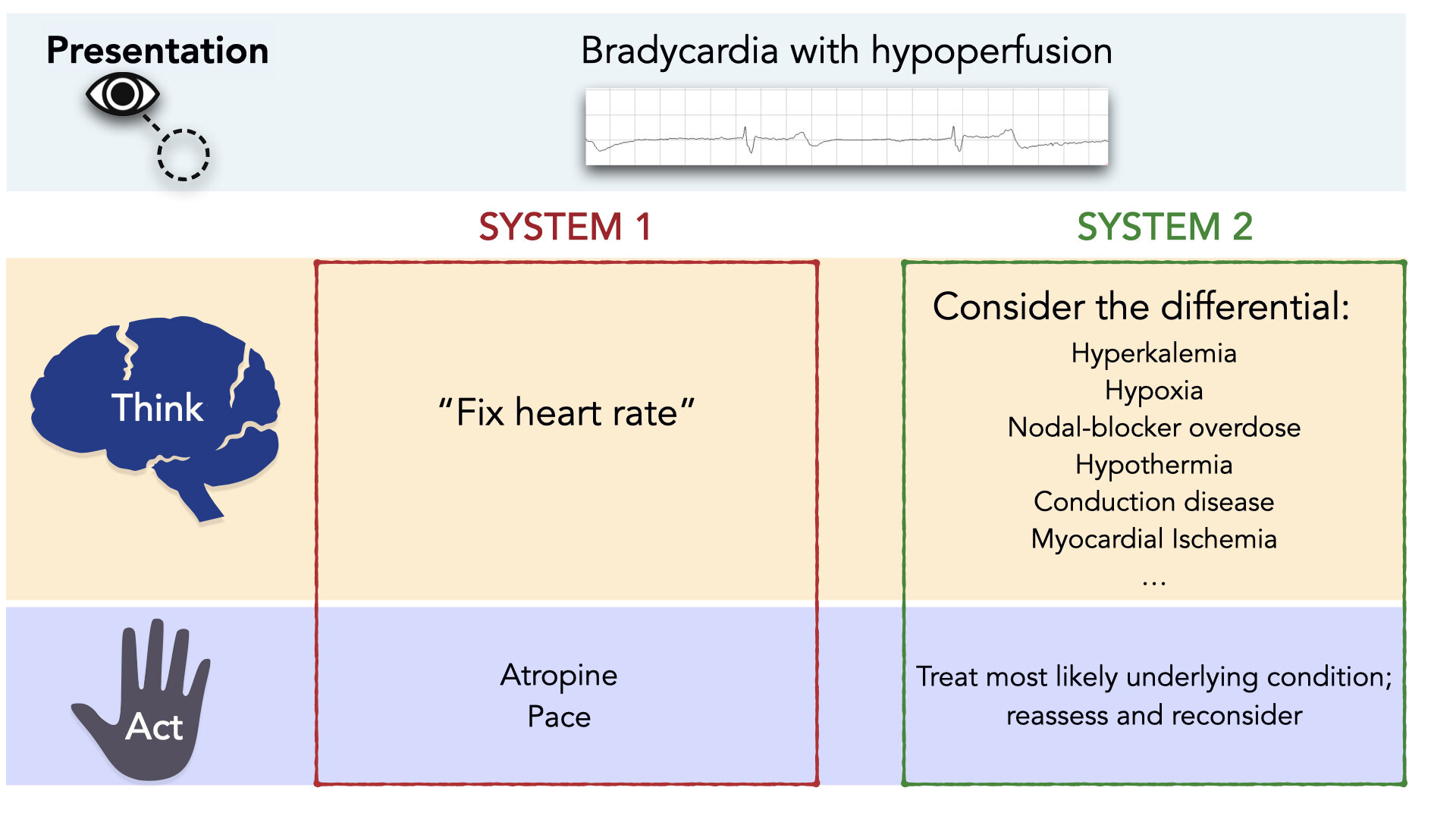
As clinicians, System 1 thinking is essential. It allows us to use contextual information and patterns to act rapidly. But System 1 thinking is far from perfect, and can be error-prone; especially in situations that warrant embracing complexity and a slower, more deliberate approach. Contrary to established algorithms, bradycardia is almost always a System 2 situation. Bradycardia is a clinical sign that has a broad differential diagnosis. [2] (Figure 1) Importantly, treating bradycardia appropriately requires consideration of this differential diagnosis.
Figure 1: Treatment of Bradycardia is a System 2 Affair.
Back to the case
Treatment of atropine and pacing-resistant bradycardia is a rare occurrence, but is one that should not be met with complete surprise, either. As discussed above, there are several causes including hypothermia, calcium channel or beta blocker overdose, and hyperkalemia which are classically resistant to atropine and pacing. Even more importantly, the causes of bradycardia can be multifactorial, such as occurs in BRASH syndrome. In BRASH syndrome, compromised renal function leads to decreased excretion of nodal-blocking medications and hyperkalemia in a feed-forward loop.
Managing patients with severe bradycardia requires thorough assessment including incorporation of the history. In addition, it requires the openness to consider alternate etiologies when the initial treatment plan is ineffective. Fortunately, the first-line treatments for calcium channel/Beta-blocker overdose and hyperkalemia are the same; calcium and sodium bicarbonate. The calcium and the sodium help to stabilize the myocardial membrane and improve cardiac myocyte function. The next go-to treatment for symptomatic bradycardia is epinephrine, either as a push-dose or infusion. Epinephrine has supplanted dopamine as the primary pressor in symptomatic bradycardia in many agencies.
On arrival to the emergency department, the patient’s labs included a pH of 7.0, HCO3 10, K of 6.9 and a Creatinine of 12 consistent with metabolic acidosis, hyperkalemia and renal failure. As we can see, the patient was quite hyperkalemic. The logical argument that her bradycardia was from her medications alone would have been incorrect. It still likely contributed to her clinical presentation especially if her medications decreased her renal function and put her into a cycle leading to hyperkalemia as occurs in the BRASH syndrome.
EMS MEd Editor & Figure Design by Maia Dorsett, MD PhD FAEMS
References:
[1] Kahneman, D. (2011). Thinking, fast and slow. Macmillan.
[2] Sidhu, S., & Marine, J. E. (2020). Evaluating and managing bradycardia. Trends in cardiovascular medicine, 30(5), 265-272.
[3] Serge Barold, S., Falkoff, M. D., Ong, L. S., & Heinle, R. A. (1987). Hyperkalemia-induced failure of atrial capture during dual-chamber cardiac pacing. Journal of the American College of Cardiology, 10(2), 467-469.
[4] Kahloon, M. U., Aslam, A. K., Aslam, A. F., Wilbur, S. L., Vasavada, B. C., & Khan, I. A. (2005). Hyperkalemia induced failure of atrial and ventricular pacemaker capture. International journal of cardiology, 105(2), 224-226.
[5] Farkas, J. D., Long, B., Koyfman, A., & Menson, K. (2020). BRASH syndrome: bradycardia, renal failure, av blockade, shock, and hyperkalemia. The Journal of emergency medicine, 59(2), 216-223.